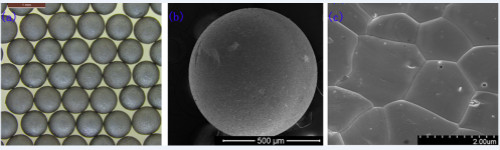
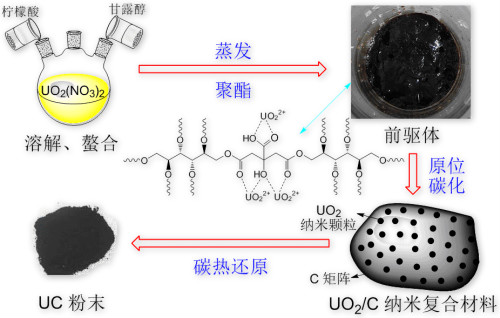
Uranium dioxide (UO2) nuclear fuel has been widely used in nuclear power plant pressurized water reactors. It has the characteristics of high melting point, isotropic expansion, good irradiation behavior and mechanical properties, but it has low thermal conductivity and easy embrittlement. problem. And uranium carbide (UC) nuclear fuel has extremely high hardness, and does not undergo phase transition in a wide temperature range, so it can withstand a high service temperature. The thermal conductivity of uranium carbide is 21.7W / (m · K) (1237K), the density is 13.63 g / cm3, and the uranium content is 95.2%, both higher than uranium dioxide. Uranium carbide nuclear fuel is considered an ideal candidate for fourth-generation reactors. Based on the exploration and research of the closed fuel cycle in the advanced nuclear energy system driven by accelerators, uranium carbide can also form a binary mixed eutectic system with plutonium (Pu) and some minor actinides (MAs). Therefore, researchers chose uranium carbide as The form of renewable nuclear fuel. Recently, researchers at the Institute of Modern Physics of the Chinese Academy of Sciences have successfully prepared UC ceramic nuclear fuel pellets using the sol-gel method combining instant-free cooling mixing and microwave heating; Single UC powder. Researchers cooperated with the Swiss Paul Scherer Institute (PSI) to jointly develop a rapid sol-gel process platform combining room-temperature instant-free cooling mixing and microwave-assisted heating, and successfully used the platform to prepare uranium carbide nuclear fuel pellets ( Figure 1). Firstly, the carbon black is uniformly dispersed in the gel agent solution (HMUR) at the nano level by the method of ultrasonic dispersion, then the C-UO32H2O gel ball containing carbon black is prepared by the sol-gel method, and finally the carbon thermal reduction The reaction transforms it into uniform UC ceramic pellets. The particle size of the prepared UC ceramic pellets is 675 ± 10μm, and the density can reach more than 92% of the theoretical density. The platform will be directly applied to the batch preparation of regenerated carbide nuclear fuel pellets in a closed fuel cycle. Researchers also use Pechini-type polymerization chelation to form stable UO22 + -CA complexes by chelating citric acid (CA) and uranyl ions (UO22 +); as the solvent evaporates and the complexes and mannitol polymerize The occurrence of the cross-linking reaction results in a loose and porous precursor material; then in-situ carbonization results in UO2 / C nanocomposite materials; and finally, UC powder is obtained through carbon thermal reduction (Figure 2). This method shortens the migration distance between reactants by uniformly mixing U and C at the atomic level, and achieves the preparation of UC powder at a relatively low temperature (1400 ° C). This work has certain application prospects for low-temperature synthesis of carbide fuels containing Pu and MAs. This research was supported by the strategic leading science and technology project of the Chinese Academy of Sciences (category A) "Future Advanced Nuclear Fission Energy-ADS Transmutation System" project and the National Natural Science Foundation of China project (design, preparation and performance research of advanced transmutation fuel elements). The results have been published in the international journals Ceramics International and Journal of the America Ceramic Society. The first authors of the article are Tian Wei and Guo Hangxu respectively. Figure 1: Instant no-cooling mixing-microwave heating sol-gel method to prepare UC ceramic nuclear fuel pellets. a: physical photo of UC ceramic nuclear fuel pellet; b: SEM photo of UC ceramic nuclear fuel pellet; c: microscopic morphology of UC ceramic nuclear fuel pellet Figure 2: Preparation of UC powder by Pechini type polymer chelation. First, citric acid (CA) chelate with uranyl ions to form a stable UO22 + -CA complex; as the solvent evaporates and the polymerization crosslinking reaction between the complex and mannitol occurs, a loose and porous precursor is obtained Materials; then in-situ carbonization to obtain UO2 / C nanocomposites; finally, UC powder is obtained through carbothermal reduction Metal Clothes Hanger,Galvanized Hanger,Galvanized Industrial Hanger,Galvanized Shirt Notched Hanger Shaoxing Jiandong Metalware Co.,Ltd. , https://www.laundry-jd.com