According to foreign media reports, an all-solid-state battery is a battery in which all components are solid. Because it can store more energy and has the potential for safer operation, it has become a next-generation battery that replaces lithium-ion batteries. attention. If mass production of solid-state batteries can be achieved, it will bring revolutionary changes to the electric vehicle (EV) industry because it can effectively increase the cruising range of electric vehicles and can significantly reduce the size and weight of electric vehicles. However, solid-state batteries will fail after cycling (repetitive charging and discharging) under actual current, which is also one of the obstacles preventing their large-scale commercialization. However, researchers at the Faraday Institution of the University of Oxford in the United Kingdom have taken measures to understand the mechanism of solid-state battery failure (a necessary prerequisite to avoid such failures). When the battery is charged, the dendritic metal lithium formed during the reduction of lithium ions is dendrite, which will continue to spread through the solid state, ceramics and electrolyte, resulting in a short circuit of the battery. A long time ago, researchers knew that the anode of a solid-state battery will produce pores, but people have not yet understood the role of such pores in the formation of dendrites. This research combines cutting-edge electrochemistry and imaging technology to basically understand the formation of pores in the battery cycle and the role of pores in battery dendrite growth and battery failure. Scientists studying solid-state batteries face two challenges: 1. When the battery cycles between charged and uncharged states, it is necessary to prevent dendrite growth. 2. The solid electrolyte and lithium anode (negatively charged electrode) will form pores during the discharge process, resulting in a reduction in the contact area between the two parts of the battery. It is difficult to distinguish the process of lithium plating and lithium stripping from batteries using two common electrodes, so the researchers of this study used a tripolar battery to study the battery cycling at the lithium metal / ceramic interface. Affect, and choose Li6PS5Cl as a solid electrolyte, such sulfides have higher conductivity than oxides, some companies trying to commercialize solid-state batteries will use it as an electrolyte. Compared with other highly conductive sulfides, the sulfides are less fragile. The researchers found that if you want to avoid the formation of dendrites in solid-state batteries, you need to control the critical current density (that is, the critical current density at which pores begin to form) during the lithium ion stripping (CCS) process to cycle the battery. This is true even if the current density is lower than the threshold when dendrites are formed during lithium plating. When the current density is greater than the current density of CCS, pores will accumulate in the battery cycle, and the contact area of ​​the solid electrolyte will decrease accordingly, resulting in an increase in local current density until dendrites are formed, resulting in battery short circuit and failure. Although there are more and more commercial solid-state batteries that are not rechargeable, such as medical implants for heart detection and so on. However, electric vehicles require mass-produced solid-state batteries to ensure that they can operate safely during the life of electric vehicles and reach acceptable performance levels. There are still considerable challenges in mass-produced solid-state batteries. Lithium ion batteries currently used in electric vehicles contain flammable organic liquid electrolytes. During battery charging and discharging, charged lithium ions will pass through the electrolyte. Such liquids have potential safety hazards. Replacing liquid electrolytes with solid electrolytes can eliminate fires. risk. Scientists all over the world are working hard to develop new battery chemicals to achieve a certain performance (power density and energy density) of the battery, so that the driving experience of electric vehicles is comparable to that of internal combustion engines. The prerequisite for the development of lithium metal anodes is the elimination of liquid electrolytes, which significantly improves battery performance. (Author: Yuqiu Yun) Led Flat Par Can ,Battery Pack,Lithium Ion Battery Pack,Power Can Light Wuxi Shengda Yukun Energy Development co.,Ltd , https://www.xlite-solarlight.com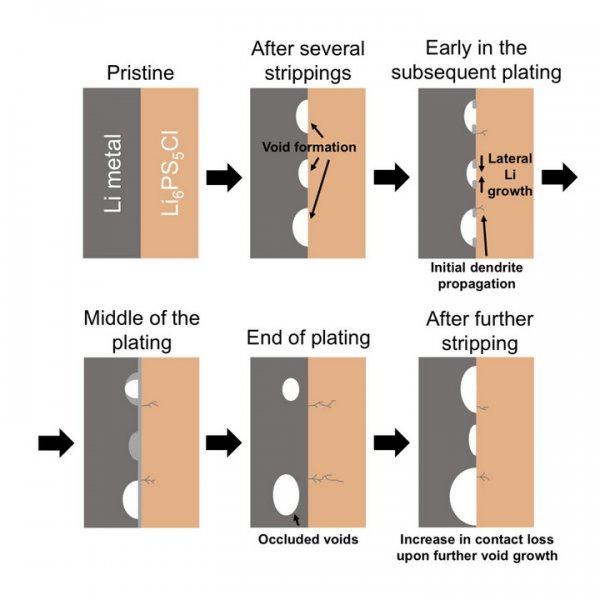
(Source: Natural Science Magazine)