* Steel Buildings are Rust Resistant, Fire Resistant, and Virtually Indestructible
All you need is an existing concrete pad or a floating foundation to get started.
Steel Structure Garage,Metal Sheds And Garages,Metal Shed Garage Building,Prefab Metal Shed Building Foshan TianPuAn Building Materials Technology Co.,Ltd. , https://www.tpa-prefabhouse.com
(Three-dimensionalall-dielectric metamaterial solid immersion lens for subwavelength imaging atvisible frequencies) 
2. Anisotropic phase separation and migration in nanocrystalline catalysts
(Anisotropicphase segregation and migration of Pt in nanocrystals en route to nanoframecatalysts) 
Niu et al. demonstrated the evolution of phase segregation in platinum-nickel rhombohedral dodecahedrons. In the initial growth phase of the rhombohedral dodecahedron which leads to the final segregation of platinum into 14 axes, the anisotropic growth of the platinum-rich phase along the <111> and <200> directions forms a highly branched, embedded in the nickel-rich shell. Rich platinum fourteen foot structure. As the growth time continues to increase, the platinum-rich phase selectively migrates through the 14 axially outward to the 24 sides, making the diamond dodecahedron a form of a platinum-rich framework surrounding the nickel-rich internal phase. The discovery of anisotropic phase separation and migration mechanisms provides a completely new approach to the preparation of nanocatalysts based on component distribution and performance requirements. (Nature Materials DOI: 10.1038/NMAT4724)
3. Chiral metal-coordination arrangement of molecules in organic framework
(Coordinative alignment of molecules in chiral metal-organic frameworks) 
Seungkyu et al. distinguished single and double bonds in gibberellin and enantiomerically crystallized racemic jasmonic acid, after which the absolute configuration was only inferred from its derivatives. (Science DOI: 10.1126/science.aaf9135)
4. Dependence of critical temperature on superfluid density in overdoped copper oxide
(Dependence of the criticaltemperature in overdoped copper oxides on superfluid density) 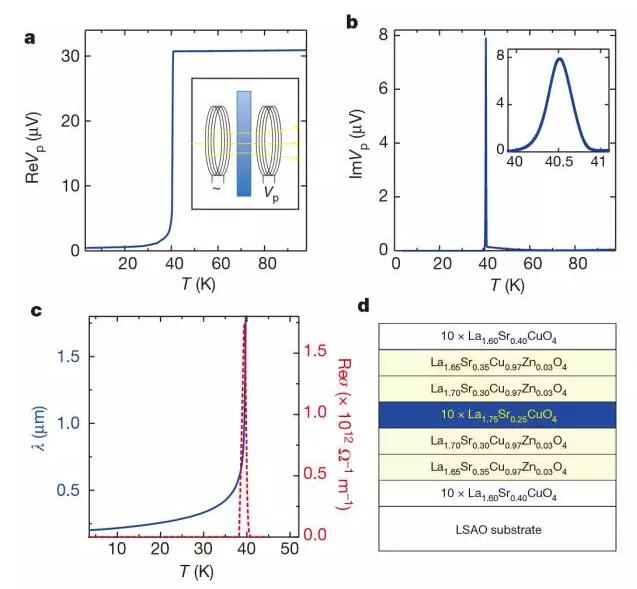
Božović et al. studied the phase diagram of the entire overdoping of La2-xSrxCuO4 and found that the magnetic penetration depth and phase stiffness depend on temperature and doping. The measured values ​​of the magnetic penetration depth and phase stiffness of the samples have a precision of one thousandth, and a large number of statistics indicate clear trends and intrinsic properties. The change in critical superconducting temperature in a homogeneous film is very small (less than 1K). The doping phase stiffness of each stage decreases linearly with temperature. The dependence of the zero-degree phase stiffness on the critical superconducting temperature is linear, but with a certain offset. However, the dependence on the origin becomes a parabola. This proportionality is inconsistent with the standard Bading-Cooper-Shriver description. (Nature DOI: 10.1038/nature19061)
5. Enhanced superconductivity in the electron-doped phosphorus group compound Ba(Fe1.94Co0.06)2As2 on the iron surface
(Enhanced superconductivity in surface-electron-doped iron pnictide Ba(Fe1.94Co0.06)2As2) 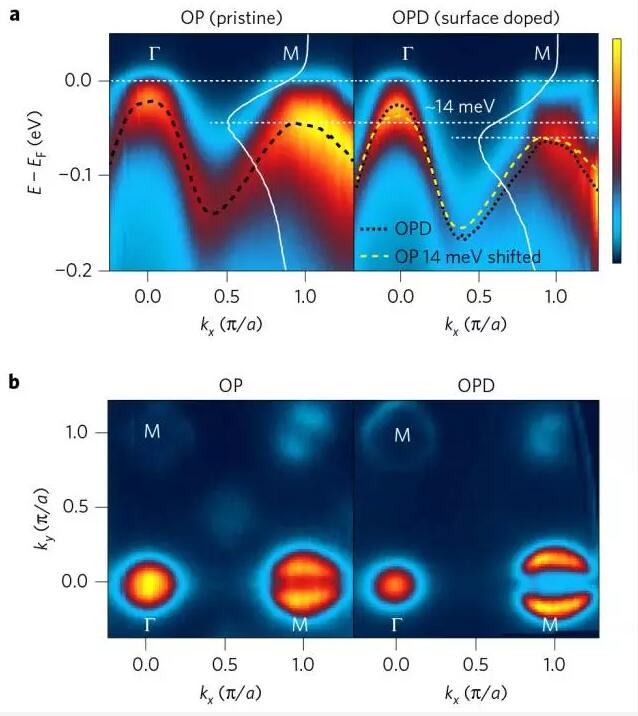
Kyung et al. reported that the use of angular resolved photoelectron spectroscopy revealed that the surface doping increased the Tc of Ba(Fe1−xCox)2As2 from 24K to 41.5K. The results of the study indicate that higher electron doping with a topological change in the Fermi surface is very advantageous for superconductivity and is advantageous not only for iron chalcogenides but also for iron phosphorus compounds. (Nature Materials DOI: DOI: 10.1038/NMAT4728)
6. Carbon-coated iron nanoparticle catalyst
(Identification of carbon-encapsulated iron nanoparticles as activespecies in non-precious metal oxygen reduction catalysts) 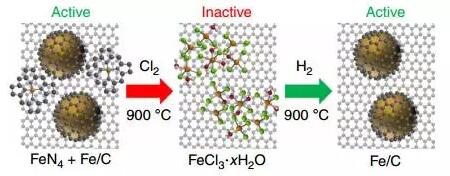
Varnell et al. recently demonstrated that treatment of an iron-based, non-noble metal catalyst with high temperature gas phase chlorine and hydrogen can achieve reversible activation and deactivation of the catalyst. In addition, they observed the heterogeneity of the catalyst as the chlorine gas and hydrogen were treated by X-ray absorption spectroscopy and Mössbauer spectroscopy. Experiments have revealed that the adjacent sites of iron nanoparticles play a major role in the activity and stability of the catalyst. (Nature Communications DOI: 10.1038/ncomms12582)
7. Active sites in iron-containing zeolites
(The active site of low-temperature methane hydroxylation iniron-containing zeolites) 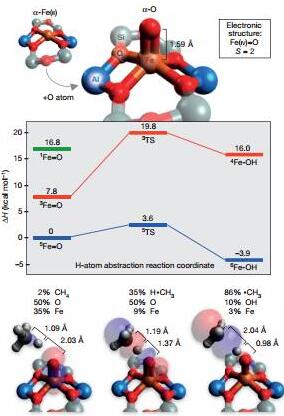
Recently, Snyder et al. reported a method of site selection spectroscopy commonly used in bioinorganic chemistry to overcome this problem. Magnetic circular dichroism reveals that α-Fe(II) is a mononuclear, highly spin, planar square Fe(II) site, while the reaction intermediate α-O is a mononuclear, high-spin Fe(iv) =O species, its excellent activity is derived from a binding coordination geometry imposed by the zeolite lattice. These findings indicate that this method has important application value in exploring active sites. (Nature DOI: 10.1038/nature19059)
8. Ultra-fast formation of thermal excitons in MoS2/WS2 heterostructures
(Ultrafast formation of interlayer hot excitons in atomically thin MoS2/WS2heterostructures) 
Recently, Chen et al. obtained the exact experimental evidence of the electron transfer intermediate state in the transition from the MoS2/WS2 interface layer to the interlayer excitation using the energy-state resolved ultrafast visible/infrared microspectrometer. The free carrier migration interface The speed is much greater than the recombination rate of the excitons in the layer. This result is a good explanation of how significant charge transfer rates and photocurrents are formed. (Nature Communications DOI: 10.1038/ncomms12512)
This article is authorized by the new material online (micro signal: xincailiaozaixian), if other media need to reprint, please contact the new material online small series (micro signal)
High grade, heavy gauge steel building kits will definitely protect you from the zombie apocalypse, but most of our customers value its rust and fire resistance just as much.
And if you`re worried about the risk of fire, you can take a breather knowing steel is non-combustible.
Unlike wood sheds or workshops, prefab steel buildings are fire-resistant and won`t feed a fire should one spark nearby.
* Steel House are Easy to Build Even if You Lack Tools, Skills, or Muscles
As long as you have a few friends, coworkers, or begrudging family members for help, you can build your prefab metal garage or shed in a few days.
We typically despatch our steel building kits within a couple of weeks - and in many cases quicker - so you can get right to work.
You don`t need to hire any special labourers, rent any expensive lifting gear, or even beef up your power tool collection to make one yourself.
"Nature" and "Science" Week (08.15-08.21) Frontiers of Materials Science
Abstract 1. Three-dimensional all-dielectric super material solid immersion lens (Three-dimensionalall-dielectricmetamaterialsolidimmersionlensforsubwa...
1. Three-dimensional full dielectric metamaterial solid immersion lens