Comprehensive review of new ways of polymer micelles and structural evolution of micelles in June 001 Chemical Journal of Chinese Universities Liu Shiyong Jiang Ming Department of Polymer Science, Fudan University and Key Laboratory of Molecular Engineering of Polymers, Ministry of Education, Shanghai 200433 Some new approaches, such as Use special interaction hydrogen bonds. Ionic interactions and polymer metal ion coordination complexes change the temperature medium environment and the induction of chemical reactions, and a brief introduction to the evolution of macromolecular micelle structures to obtain nuclear crosslinks and shell crosslinked micelles is presented. In recent years, the field of supramolecular structure and assembly of nanofluids has attracted widespread interest. 3. High scores, the formation of assembled molecular aggregates through non-chemical 7 bonds, the corresponding research to obtain high orderly structure The control of molecular aggregates and their structural functions and the design of new materials have played an important role. 13 This enables size-controlled nano metal clusters and semiconductors as well as high-fraction nanofibers and nanotubes. Mu Wen focuses on the self-assembly of polymers in Loewe. First of all, we will introduce the block copolymers or graft copolymers, and then we will review various new ways to polymerize the micelles. Finally, the self-assembly based on high-produced micellar structure is briefly introduced. 1 Classical polymeric micelles 1.1 Micelleization behavior of block copolymers and graft copolymers in selective solvents Block copolymers and graft copolymers produce phase separation between different blocks or graft chains. Various phase structures occur spontaneously. In the solid state, this microphase separation phase structure has been studied very well. Depending on the molecular weight of the block, the composition of the copolymer, etc., the block copolymer can form a spherical, spiral, and layered structure 4. This article focuses on the formation of block or graft copolymers in selective solvents or mixed solvents. The molecular assembly structure, polymer micelles. Polymer micelles can be divided into water-soluble micelles and organic solvent micelles according to different solvents. According to small molecule surfactants, the former is a conventional micelle, and the latter is a reversed micelle. According to the structure of the micelles, the stars of the star-shaped micelle bundles are small and the shells are relatively large. The theoretical treatment of the block copolymer micelles is based on thermodynamic considerations, namely that the pseudoaggregates of seven aggregates and molecular chains reach an equilibrium state. The micelles and small molecules of the small molecule surfactant are in dynamic equilibrium, and the polymer micelles exchange the micelles and the free polymer chains very slowly because the viscosity of the segments in the micelle core is extremely high. Polymer cores, such as polystyrene, are in the glassy state, on a time scale scale of 31. High-dimension broad micelles; species are frozen in structure. Geng et al. reported that graft copolymers with multiple short-chain branches in the selective solvent contributed to intramolecular aggregation to form monomolecular micelles. Change the nature of the solvent, that is, choose to pay. The chain or graft chain can be selectively dissolved in the solvent, respectively. , et al.21 successfully immobilized the middle chain micelles of the block copolymers with photocrosslinking and then passed through; where they separated and proceeded. 1.2. The classical preparation method of polymer micelles The traditional way to prepare polymer micelles is based on the use of selective solvents, which allow the insoluble blocks to form micelle nuclei and the other blocks to form micelle shells. The method is to block or Graft copolymers dissolve in a common solvent and then add a precipitant to a block or graft chain. Also improve the dialysis or evaporate the original joint agent. Make the nature of the lotion a strong, undesirable agent for a certain block. ; The father of the ... ... 0 prepared by the 1.81 is the polybenzocidebu propionate. , 54, 8 segments relative to the corpse. A very long precedent of coprecipitate is added to the precipitation of 1 block of water. After dialysis to remove the preparation was received a date of 2000 ye 622. Fund Projects National Natural Science Foundation Grant No. 29992590, 59773023 and the National Fundamental Research Section Fundamental Research Funding for Macromolecular Condensation. Contact Profile Jiang Ming was born in 1938, male, professor, doctoral tutor, mainly engaged in polymer physical chemistry research. The micelles can also be used in this way, such as polyisoprene prepared in equimolar 24, cinnamoyloxyethyl methacrylate, 11 capsules, and micelles in hexane solvent. Another method is to dissolve the block copolymer directly in a single solvent or in a mixed solvent, which may require heating during the reaction. This method is generally only effective for preparing star micelles. 2 A variety of new ways to polymerize micelles A new way to achieve high-yield micellization includes a variety of physical and chemical processes. The temperature-dependent chemical reaction of the dissolving power of the intermolecular specific interaction block and the adjustment of the medium environment can lead to the formation of polymer micelles. Obviously, these micellization processes go beyond the traditional micellar system that only depends on the selective solvent used in the plant. 2.1 Special interactions and the spatial effects of block arrangement 7,5. The micellization behavior of polystyrene 4,4 guanophenicol 134 in toluene was observed. Here, benzene is a poor solvent for the tundish, and it is inert to hydrogen bonds. Therefore, there is a strong hydrogen-bond interaction in the toluene between the phenolic OH groups of the tray, which acts as an additional driving force for the nucleation of the glue card. The resulting glue is columnar. Ruoshi, Chu et al. recently reported that 1; block copolymers of instar pulverized polypeptide chains and polyethylene oxides are synthesized in water to form fibrous micelles, which solves the problem of water solubility of called peptides. The core of the micelle is a powdered polypeptide. It also becomes a driving force for micellarization with its own, key weight. 1816 et al.27 studied reversed micelles formed by polystyrene acrylate block ionomers in organic solvents such as toluene, in which ion-ionic interactions between ionic groups in the polyacrylate blocks also become Additional driving force for micelle nucleation. Generally, the block copolymers of the mortar are all composed of rudimentary segments. If the two segments are respectively composed of rigid and soft parts, the segments of the first segment of the mountain are connected by amine bonds. In fluoroacetic chloride and blend solvents, fluoroacetic acid can quaternize the atoms on the quinoline so that the polyphenylquinoline blocks become more rigid polyelectrolytes. When fluoroacetic acid is the main component, micelles having polystyrene as a core, polyphenyl, and morpholine as a shell are formed. Due to the high rigidity of the polyphenylquinoline block molecular chain, this block is arranged radially when the spherical micelle shell is formed, and the size of the flexible polystyrene segment is limited. + can be extended to the interior of the nucleus, so the inside of the micelle is hollow, and the size of the micelle reaches the number of aggregates on the order of micrometers (1), becoming the most human synthetic molecular gel of the size reported so far in the literature and the Shanke block. The amide bond between the molecules forms an intermolecular hydrogen bond and has a stabilizing effect on the micelle structure formed. This hollow type micelle can dissolve human fullerene. Document 31 also reports on the effect of varying the temperature on the micellization of the polymethylentrozine 4 silicolane stilbene ferrocene copolymer due to the fact that the polyferrocene block 2.2 has a considerable portion of water-soluble polymers such as polyisopropyl acrylamide, human, poly. Vinyl methyl ether, polymethylacrylic acid, and antimony ethyl ether esters are heat-sensitive in aqueous solution and have a few phase behaviors. For example, if the temperature exceeds 391, phase separation occurs; if copolymerized with other water-soluble monomers, such as acrylic acid and acrylamide, the phase separation temperature can be adjusted. Based on this, Ding et al.6 studied the polyacrylonitrile-propylene propene to ethylene oxide. 1. Micellarization was caused by the temperature change in the water and liquid. 1 et al. 4 reported that 1 阽 7 cations can form single-stranded core-shell micelle structures in water by changing the temperature and controlling the heating rate. For the book and state, the composition of the block copolymer also observes temperature-sensitized behavior. The other 37 extensions studied poly(methyl acrylate), methylamine ethyl ester 4 cyanomethacrylate 2 pairs. W inflammation LL ring M B Jl! According to the micellization behaviors of PDMAEMA4MF and V-water, the PMEl homopolymer has a phase behavior of 3445 at Pl7, depending on the molecular weight. Exceeding this temperature results in the formation of a micellar structure in which 1 person is a shell and 1 is a core. The human, shell progress cross-links can prepare the core of the remaining water hydrophobic components adjustable vertical cross-linked micelles. 2.3 Change of out-of-straight or ionic strength In one or two blocks of block copolymers or graft copolymers, one can metabolize into units capable of trapping protons, a classic example; 笈2 Vinylpyridine When the value of the aqueous solution of 100 million rings changes from 1 to 1, the poly- 24 alkenylpyrazole block changes from a dissolved state to insoluble, resulting in micelle formation. 38. For polymethacrylic acid, ethylaminoethyl The elbow elbow precipitates from the aqueous solution, whereas the human block is almost unaffected by the ionic strength in the case of dissolution in water, but at 1 yo. Under the conditions, it can be dissolved, and when it is estimated to be as high as 8, the meaning can be precipitated. The use of two blocks of non-conjugation to change the pre- and ionic strength, that person 4, the two blocks of yttrium can each become micelles and micelles 1. 2.4 Ionic interactions using polyelectrolytes Under certain conditions, polyelectrolytes and oppositely charged surfactants can act to form polyelectrolyte surfactant complexes. Such as 4, urethane polyethylene oxide 1 sodium acrylate, block copolymers and thiophene pyridine blood glucose forming a micelle-like structure, the formation of aggregates narrow distribution, with a spherical structure. When the polyelectrolyte with opposite charge is mixed, a polyelectrolyte complex can be produced. When a block polyelectrolyte, ie, a block copolymer containing a polyelectrolyte chain and a polyelectrolyte homopolymer with an opposite charge are mixed, a polyelectrolyte is formed. The complex is a core that dissolves in water-soluble micelles with shells that do not contain any charge. 1 et al. 444 paid this systematic study. Examples are polyethylene oxides, pi-polyethylene oxide, polybutylene, 1-aspartic acid polyethylene oxide 44 sodium methacrylate, 4-ethyl allyl pyridinium salt 142, polyethylene oxide VII, Proline ribonucleotide 143 epoxide, 1, and aspartate chymotrypsin etc. 144. The earliest examples of the formation of such micelles were 181 et al. The micelles formed in the mixed organic solvent of vinylpyridine pyruvate styrene blend system, in which the polyelectrolyte complex formed of polyacrylic acid and poly-4-enyl pyridine constitute the core of the micelle, and the polymethylstyrene molecular chain forms Micellar shells. 15 Hydrogen-bonding Induced Micellization We found on the basis of long-term studies on the compatibility of multi-component polymers that the introduction of a functional group in a blend system without special interactions can make the incompatibility high. The molecular blend system achieves incompatible compatible chelation transitions, and the conversion between isolated molecular coil aggregates and macromolecular complex aggregates is achieved in solution. We have summarized the systematic research results in this field. 146. These complexes precipitate from the solution and have an irregular structure. Although the vast majority of polymers contain or can introduce specific interacting groups, resulting in strong intermolecular forces, the assembled methods to form ordered structures have been limited. Based on our research on block copolymers and macromolecule complexes for many years, a new approach to capture micellarization was developed. As previously stated, for the micellization of block copolymers, the solvent is chosen so that the insoluble blocks are aggregated into the core and the soluble blocks form the shell resulting in the stabilization of the aggregates. The proposed hydrogenation through hydrogen bonding leads to micellization and This mechanism is completely different 47. The basic idea is to homopolymer or random copolymer, 1 into the 8 block copolymer in a non-selective solvent solution, if. Americium, which leads to humans, helium, but the presence of a soluble block 8 promotes the stabilization of aggregates, eventually forming human, shell-like micelles. 1 is an example, where the block copolymer contains, 3, and Heheren diblocks, wherein Hehe Renke and Random Copolymers, 50-finger-containing, He-20-indented, and group-modified polystyrene. Hydrogen-bonding interactions form complexes 46. Dynamic and static light scattering Non-radiative energy transfer Fluorescence spectroscopy and viscosity methods confirm that the macromolecule assembly is a micelle with a spherical structure with an average hydrodynamic radius of 70 to 10, 30 bases. The increase, poor key effect, strong. The number of micelle aggregates increased, the density increased but the size became smaller 4748. Similarly, we also studied the block graft copolymers of polystyrene heptaene octadecene methacrylate ethyl methacrylate pieces 1 and 50 in toluene. Glue-bonded chlorentoside has been used for some researches. This, 8. The micellization caused by the interaction between the system and the system is a new way of molecular assembly. 26 Coordinated sites of metal ions and polymers are reported in (3). Chlorine-doped platinum and beta-oxime are reported. Dingchimen is used in aqueous solution and polybutadiene, aspartic acid The coordination between the phases forms micelles. Among them, the water-insoluble complex formed with poly-l-aspartic acid constitutes the core of micelles, and the polyethylene oxide that is solved 4 forms a shell of micelles. This micelle is stable in water. However, the salt solution 4 in 37 slowly releases 0, thus having potential application as a tumor-targeted drug carrier. 27 The phase separation of polymer blends in solutions induces micellization. If, between the polymer- and phase-center-blend solutions, the phase separation of the products is also based on this principle and other reports are reported in the polystyrene, chlorinated isoprene inlays. The addition of polystyrene homopolymer to the toluole solution of the segmental polymer induces micelle formation. The core of the micelle consists of polyhydrogenated isoprene blocks. The volume and mass of fluid of the micelle do not vary with the concentration of polyphenylene. However, the proliferation of micelles is affected by the increase in the viscosity of the high-molecular polystyrene chains. 28 Induction of chemical reactions This is a relatively new development in polymeric micelles. Qiao et al. Designed and synthesized poly-p-methylstyrene sulphaphenyl vinyl sulphoxide to seventy eight. Among them, eight chains have long been confirmed in the heating , can be converted to polyacetylene, using dynamic light scattering to track the change of the solution of the copolymer with heating, found that, due to the conversion of 50 into insoluble polyacetylene blocks, the copolymer is assembled into polyacetylene as core 8 shell 306,1 micelles. This is an example of a 7-reaction-induced micellization. A common feature of the various micelles of the 29 core-shell shells linked by secondary bond interactions to the discussion of the block-forming graft copolymers is that between the core and shell of the micelles The price keys are connected. Recently, based on the long-term study of hydrogen-bonding complexation, we proposed a new kind of micellization process, in which there is no covalent bond but only hydrogen bonding between core and shell. A specific example is to first mix polystyrene 05 with a terminal carboxylic acid group and poly 4 ene alkylpyridinium 7 in chloroform, and interact with a hydrogen bond between the plant carboxylate and the pyridyl group. Between 05 and, a graft copolymer 5455 with a main chain 3 as the graft chain can be formed. Toluene is added thereto, and it can not be aggregated due to the presence of 1 toluene, but there are special interactions between it and 015. As well as the dissolution of 15 chains, it did not precipitate. Therefore, the micelle structure formed by the solution in the core of the shell is assumed to be a spherical, and the distribution is very narrow. In the solution can be stable for a long time. There is no covalent bond between the micelle nucleus shells. It is expected that a variety of new structural molecular assemblies can be formed through the advancement of chemical crosslinking fixation and core-shell separation. In order to prepare this kind of core-shell interactions and 7-bonded micelles, we have also developed a micellarization approach with a simple history. Simply put it. If there is a special phase effect between the polymer and the polymer of the endocaponic acid, the solution is dropped into the solution. If the latter is the former's precipitant, the person will gather. However, due to the existence of hydrogen bonds or ions in humans, human aggregates will stably leave due to the presence of 8. Finally, micelles with an artificial core 8 shell were formed. This method 1 in partially nitrated polystyrene, 4-hexylidene 1 set 457! Carboxylic acid polystyrene 4 polystyrene sulfonic acid sodium 1 said 5 kg 4 and styrene ear-based acrylic copolymers etamerpyrolidone wide and many other systems to achieve, especially in the latter two systems Shan Yushi, 55 can Dissolved in water, the final formation of chemical micelles between the core and shell, and can be stable in the aqueous phase, micelle size in the tens of nanometers. The more potential application price is through the selective dyeing of the nanomicelle core and shell. We have confirmed the colloid structure using transmission electron microscopy. Obviously, this method of preparation is particularly simple. However, the morphology and size of micelles are controlled by the dynamic conditions of the preparation. 3 Structural Evolution of Polymeric Micelles The micelles formed in solution are unstable and change with changes in the external environment such as the temperature and value of the solvent. Therefore, fixing the micelle structure is an advancement in the use of such nanostructures. Ordered structure of molecular aggregates and prerequisites for the development of functional materials. This 1 in recent years, 4 points, 1 physical chemist's close attention, we here mainly introduce the recent on the micellar structure 3.1 nuclear cross-linked micelles, etc. In this area made a large number of characteristic work is mainly to use the included coffee The block copolymers of humans in which 10 of the cinnamoyl hydrazide radicals were exposed to ultraviolet light to generate double bond adducts. Therefore, the structure of the rubber camber can be easily fixed. For example, 81 gamma is formed in a towel or 0-oxo six-ring mixed agent, forming a nucleus of micelles. Depending on the composition of the diblock copolymers, asterisks or micelles can be formed, and photocrosslinking can fix these structures. For the micelles formed in 1st, 1st, and 10th-nuclei with 10纟16 as the core, the ozonized degradation micelle shell 1 can form nanoballs with aldehyde groups on the surface, and can be improved. Functionalization of Microspheres by Reaction of Aldehyde Groups 62. Porous nanomicrospheres can also be obtained from similar pathways. This porous sphere can be used to capture traces of polycyclic compounds in water. 14. Document 65 studies polyethylene oxides, lactic acid, 04 people, where the 0-end with acetal groups, human end with a Alkyl acrylate groups, in the water to form an anthropogenic nuclear glue, can be used to immobilize the micelle structure. The acetal group is then converted to an aldehyde group. It is possible to obtain functional micelles with reactive groups (4) on the micellar side. 3.2 Shell cross-linked micelle shells Cross-linked micelles are a relatively new lesson, it is similar in structure to dendrimers, has a functional group around the core has good penetrating dimensions in nanometer order and synthesis is simple, etc. Features. It is generally believed that it integrates the dendrimer hollow; 1. Ordinary micelles and emulsions leave a wide range of characteristics.士7, 3.1.1 took 715 to do the best job in this area. 7, the first research, the formation of the block copolymer, the cross-linked rubber first with quinone methyl styrene 7 block quaternization, block copolymer with unsaturated double bond in, 10 = 3 The dehydration can form a spherical micellar structure with a 1 block shell that is quaternized with light quaternary ionic liquid. However, it can polymerize the double bond in the water and a couple of surfactants to obtain a water-soluble shell crosslinked micelle. The structure can be confirmed by various means such as NMR fluorescence spectroscopy. Changing the degree of quaternization of the block copolymer composition and the hydrophilicity and hydrophobicity of the quaternization agent can effectively adjust the size and stability of the shell cross-linked micelles produced. 67 for, 3 spheroids formed in water When the micelles are cross-linked with two polyethylene oxides with primary amino groups, the resulting shells have the characteristics of a hydrogel, which can fully swell in water and shrink in dry state. If, from the micelles formed by 14 people in the water, cross-links, amino groups, 0 cross-links, and then use ozonation to degrade them, the nano-hydrogel nanospheres can be formed. In the control of the release of organic substances such as capture and release of blood, CAMEX offers extremely beneficial advantages. Clever 1 that the block copolymer 7 acetonitrile mixed solvent can form, 3 is a nuclear, shell spherical micelles, the use of photocrosslinking can be obtained in the 3, the good solvent toluene and are relatively stable Shell cross-linked micelles 71. Use, 1 seven coffee in the alveolar vesicle-like micelles, ultraviolet light according to 1; make 1 shell cross-linked and then stinking degradation of 1 segment, can form a hollow cross-linking,笕 笕 笕 笕 笕 , , , , , , 梢 é‘ é‘ é‘ é¥é¨ é¥é¨ é¥é¨ é¥é¨ é¥é¨ é¥é¨ é¥é¨ é¥é¨ é¥é¨ . . . . . . . . . . . . . . . . . . . . . .. . In the order 1 noisy methanol volume fraction between 5 off 99 can form spherical micelles, 1 for the nucleus, in the middle, He Ren, 18 people in the outermost layer. Photocrosslinking, chitoes, crunchy keratin, slow pupa, to pupa, nucleus, can form hollow nanoparticle. This nanoparticle can be used to load Rhodamine 4 model drug. The composition of PI PCEMA4hPtBA block copolymers was adjusted. In the methanol solvent, columnar micelles with PI as the core, outwards as the sequence, and scorpion tears were formed. Cross-linked intermediate layer, add people, and then ozonation degradation, 1 nuclear, hollow nanotubes can be formed. Such nanotubes can also be used to load models of drugs such as Rhodamine 1 which make full use of the progressive chemical evolution of block copolymer micelle structures, resulting in a series of functionalized nanomaterials. In order to reduce the aggregation of micelles caused by the reaction between the micelles, the reaction mainly occurs in the micelles, and the shell cross-linking reaction should proceed at a very dilute concentration of 5, which makes it difficult to practically apply the 4 cross-linked micelles. . 1.1879 From the 1 block, the formation of the nuclear quinone effect can prevent cross-linking reactions between micelles. A typical example of shell cross-linked micelles capable of producing high-I ancient content is 10 PDMAEMA PMEMA 74. In its aqueous solution, Vj molecular salt forms a nuclear colloidal structure of PM, MA, lAKMA, and KU, and then crosslinks. The pill thief enters the middle layer, can obtain the shell cross-linked micelle with the mass fraction up to 1 off. 7. Enters 73 stations to report the two shells 1 shell cross linking glue that the nuclear and the shell are respectively aiming at the electric power Yang Yang.
Epoxy Anti - Static Level Coating
Scope of adaptation:
Generally 5 years or more
Technical index:
project
index
Drying time
Table dry
≤4
Hard work
≤24
Adhesion (grade)
≤I
Pencil hardness
≥2H
Impact resistance
40 Pass
Flexibility
3mm Pass
Wear resistance(750g/500r,weightlessness,g)
≤0.03
Water resistance
48 hours no change
Resistant to 10% sulfuric acid
56 days no change
Resistance to 10% sodium hydroxide
56 days no change
Gas-resistant, 120% "
56 days no change
Surface resistance
105-108
Volume resistance
105-108
Performance characteristics:
1, excellent anti-static lasting effect, the use of static black carbon fiber manufacturing,
2, dust-proof, moisture-proof, wear-resistant;
3,Smooth surface.
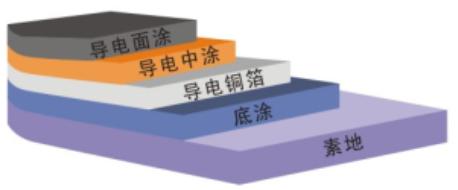
1,Base surface treatment;
2,Brush seal primer
3,Laying copper foils
4,Trowel coated epoxy conductive coating
5,Grinding, vacuuming
6,Roll coating anti static epoxy coating
Epoxy Anti - Static Level Coating
Epoxy Anti - Static Level Coating,Anti-Static Epoxy Topcoat,Epoxy Topcoat Material,Epoxy Finish Antistatic Material,Epoxy Antistatic Self-Leveling Floor
Jiangmen Kasole Building Materials Co., LTD. , https://www.kasole-paint.com